| Information | Description |
|---|---|
| Applications | Selection and implantation of skin staples depend on the patient’s condition, surgical experience, surgical technique, and wound size. |
| Mode of Action |
|
| Sterility |
|
| Storage |
|
| Disposal |
|
| Contraindications |
|
| Warnings |
|
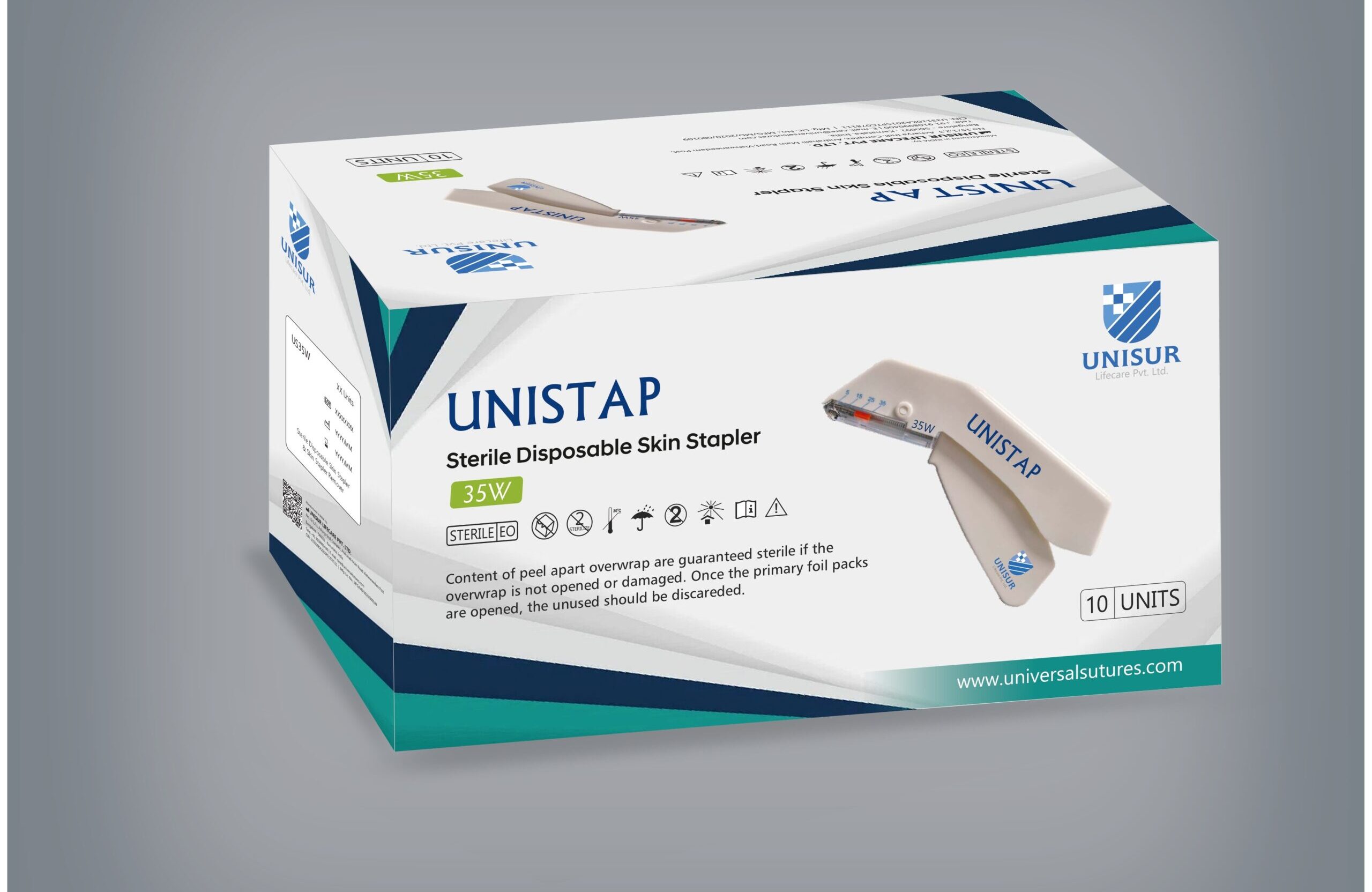
Surgical Disposable Skin Stapler (35W)
The UNISTAP is a lever action disposable skin stapler providing excellent staple visibility and comes equipped with 35 wider staples. This stapler is used in surgical procedures for skin closure. We manufacture UNISTAP skin staplers using high-quality medical-grade materials under strict quality control.As a trusted exporter, we supply UNISTAP skin staplers to hospitals and healthcare providers worldwide.